Molecular clusters play a variety of roles in our atmosphere. One is in the formation of atmospheric aerosol particles. These particles are important because (1) they reflect and absorb solar radiation and thus influence climate and the global energy budget, (2) serve as sights for heterogeneous or aqueous phase chemistry, and (3) present significant health issues in certain environments.
One mechanism for aerosol formation in the atmosphere is homogeneous nucleation, in which a supersaturated vapor forms particles literally “out of thin air”. Small molecular clusters are necessarily initial steps along the route from gas phase molecules to micron-sized aggregates and their physical-chemical properties exert a strong influence on particle formation rates. Understanding the complex mechanisms for aerosol formation remains a major difficulty in climate modeling.
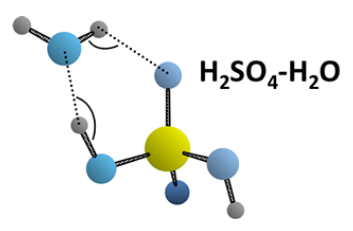
Simple acids like H2SO4 and HNO3 are active in aerosol nucleation and, therefore, our studies of acid hydrates provide basic information about aerosol precursors. Once an acid ionizes, its ability to stick to additional binding partners changes. Thus, it is of interest to address the question of how many water (or other) molecules are needed to ionize a strong acid. Typically, for acids that readily ionize in bulk solution, the number is in the 3-5 range – one or two is not enough. For example, show at the right is the structure of the H2SO4−H2O complex that we determined from gas phase microwave spectra It is clearly a hydrogen bonded system. For HNO3, we have examined its mono-, di-, and trihydrates. Even three molecules is not enough to induce ionization.
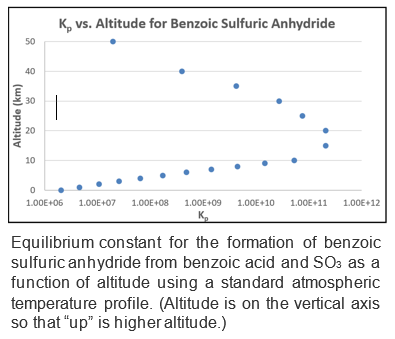
Most recently, we have been interested in the potential role of “carboxylic sulfuric anhydrides” and have reason to think they may be important in aerosol nucleation. (Emphasize “may” here, as this is not yet certain.) We discovered these systems by accident and found that they form via a heterone reaction between SO3 (and important precursor to atmospheric sulfuric acid) and carboxylic acids (which are prevalent trace gases in the atmosphere). See the "Publications" page and the "Research/Carboxylic Sulfuric Anhydrides" tab for some more info and some papers on this. Note that we have also calculated the equilibrium constants for their formation. Shown at the left is a plot of the equilibrium constant for the formation of benzoic sulfuric anhydride as a function of altitude using a standard atmospheric temperature profile. The equilibrium constants are large and we estimate that, under some conditions, these systems could be viable seeds for nucleation. We also have reason to think that they may be superacids, thus reducing the cluster size needed for ionization.