Microsolvation
Solvation exerts a huge influence on chemical reactions and, in some case, even promotes chemistry that does not happen in the gas phase. An excellent example is the transfer of a proton from an acid to a base. Anyone who has taken General Chemistry is familiar with the ionization of a strong acid in water, e.g.,
HNO3(aq) + H2O(l) —► H3O+(aq) + NO3-(aq)
Yet, it is well known that a single water molecule attached to nitric acid, or most any other strong acid in the gas phase, is a hydrogen bonded complex. Proton transfer does not occur. This begs the question, “How much water is needed to ionize a strong acid?
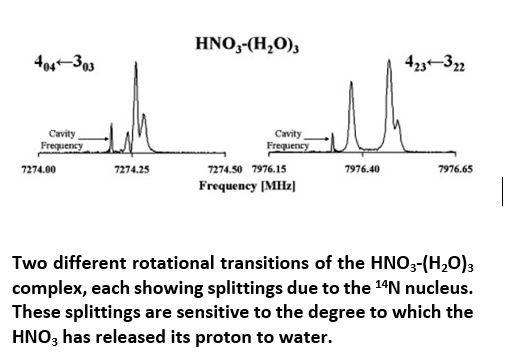
We take a cluster science approach to this problem by investigating complexes of acids and superacids with increasing numbers of water molecules. We use nuclear spin as a probe of molecular electron distribution to assess whether or not an acid has lost its proton. For example, shown at the right is a portion of the spectrum of HNO3-(H2O)3. The splittings that are visible are due to the I = 1 spin of the 14N nucleus interacting with the molecular electric field gradient. These splittings are sensitive to the electron distribution and hence to the degree to which the HNO3 has transformed into NO3- within the complex.
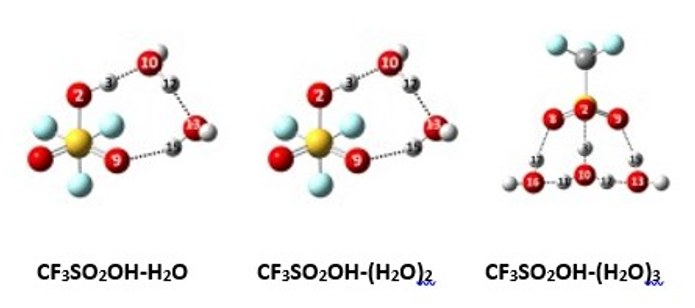
Even with the superacid “triflic acid” (CF3SO2OH), one or two water molecules are insufficient to induce ionization. However, with the third water molecule, the system becomes a CF3SO3-…H3O+ ion pair solvated by two water molecules. Shown below are the lowest energy structures of the mono-, di-, and trihydrates of triflic acid, and our spectra fully support this prediction. (You can see the hydronium ion at the bottom of the trihydrate structure.)