Proton transfer is probably the simplest and most ubiquitous of chemical reactions, occurring in diverse environments ranging from living systems to the atmosphere. Our studies seek to elucidate the factors that allow (or disallow) proton transfer. In particular, we’re concerned with both the identities of the Brønsted-Lowry acids and bases themselves, and the role of neighboring molecules in stabilizing the charge separation (microsolvation).
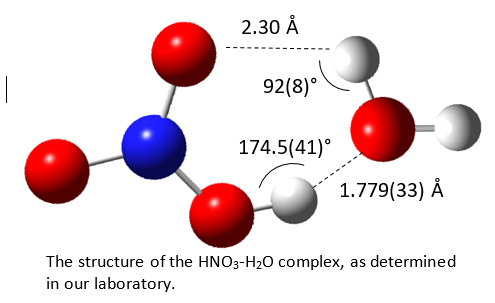
There is much experimental and theoretical evidence indicating that proton transfer within an isolated 1:1 acid-base complex in the gas phase does not occur, even when the acid and base readily undergo proton transfer under bulk phase conditions. For example, for strong acids such as HCl or HNO3 which readily ionize in water, the 1:1 complexes H2O-HCl and H2O-HNO3 are hydrogen bonded with little to no evidence of proton transfer. Shown at the right is the structure of the H2O-HNO3 complex, which is clearly a hydrogen bonded system. Even with NH3, a stronger base than water, proton transfer does not occur and the 1:1 molecular complexes remain hydrogen bonded. This begs the questions (i) How many near neighbors are required before proton transfer can take place, and (ii) What are the factors that influence the propensity for proton transfer?
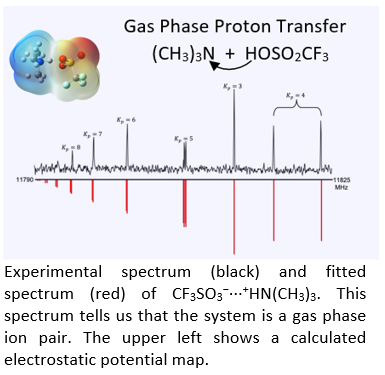
We are interested in using complexes of superacids to address these questions. Superacids have a proton donating ability that exceeds that of sulfuric acid allowing us to observe proton transfer in small molecular complexes. For example, triflic acid (CF3SO2OH) is 100 to 1000 times stronger than H2SO4 and thus its complexes can undergo proton transfer even when those of other acids do not. Shown at the left is a spectrum of the 1:1 complex formed from triflic acid and trimethylamine. Our spectra, used in conjunction with high level computational methods, unequivocally demonstrate that this system is best described as a trimethylammonium triflate ion pair: CF3SO3----+HN(CH3)3 and simple energetic arguments can be made to rationalize this result.
For more on the question of how many near neighbors are required before proton transfer takes place, and on the role of superacids in addressing this question, see the tab on “Microsolvation”.