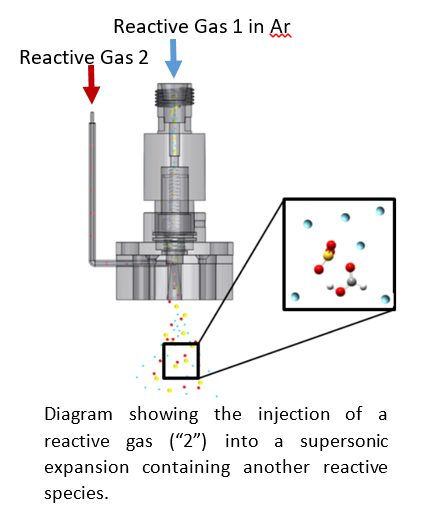
Supersonic Expansion
We use the well known (but nonetheless remarkable) technique of supersonic expansion to produce cold molecules or molecular clusters in a supersonic jet. The idea is that gas is expanded through a small orifice (or “nozzle”) from a region of high pressure (typically several atmospheres at room temperature) into a region of low pressure (typically 10−5 torr). This adiabatic expansion cools the gas to ~2 K and, at such a low temperature, the molecules begin to condense. However, the condensation stops when the expanding gas becomes so rarefied that there are no longer any collisions. What we’re left with are ultra-cold molecules and clusters in a collision-free environment, which allows us to study them without the interfering effects of a condensed medium.
The low temperature also has the advantage of simplifying spectra by putting most of the molecules in only the lowest few quantum states. Thus, we only see transitions out of these low quantum states.
To study complexes on the brink of chemical change, we frequently mix gases that would react under bulk conditions. This has to be done quickly to avoid reactions leading to solids. We accomplish this by expanding one gas seeded in an argon carrier and injecting the second gas into the expansion through a small hypodermic needle. Molecules have only a few tens of microseconds to interact before they enter the collisionless regime and, therefore, cannot react further. This allows us to prepare clusters of reactive species without producing bulk phase products.