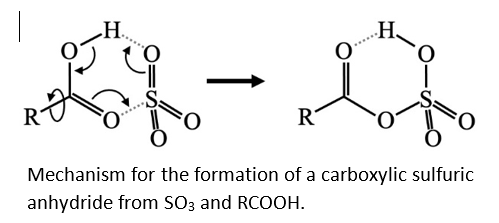
We have discovered the sulfur trioxide (SO3) readily reacts with carboxylic acids (RCOOH) to form what we have called “carboxylic sulfuric anhydrides”. The reaction is shown at the right. Our interest in this arose because SO3 and carboxylic acids are important atmospheric trace gases. SO3 is the precursor to sulfuric acid, and carboxylic acids had been postulated to assist in its conversion to H2SO4. What we didn’t expect was that the reaction at the right takes place with very small and sometimes negative activation barriers.
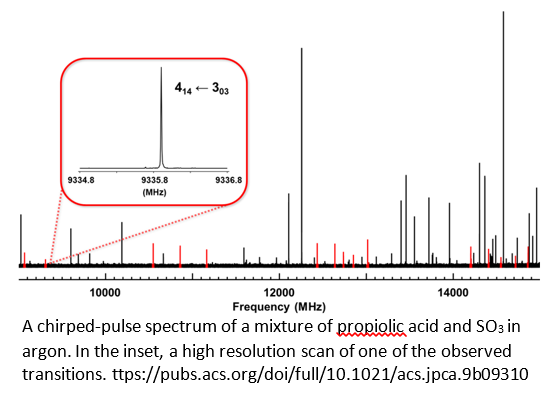
Our initial observation involved formic acid (HCOOH) but we have since demonstrated that the reaction takes place with a variety of carboxylic acids. For example, shown below is a chirped-pulse spectrum of a mixture of SO3 and propiolic acid (R = HCC-), with a high resolution trace of a single transition in the inset (https://pubs.acs.org/doi/full/10.1021/acs.jpca.9b09310).
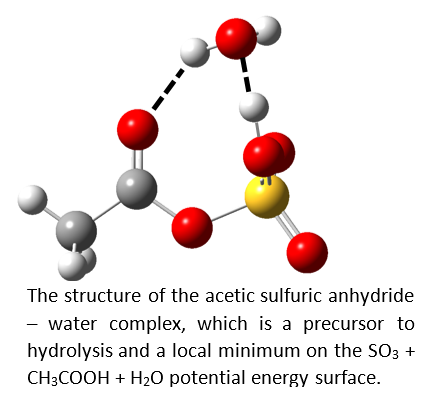
Another interesting question is “How much water is needed for the hydrolysis of these anhydrides?” This is somewhat parallel to the question of how much water is needed to ionize a simple acid (see the Research/Superacids and Proton Transfer tab.) We have also investigated the water complex of acetic sulfuric anhydride (left) and captured this local minimum on the potential energy surface. (The global minimum is the CH3COOH-H2SO4 complex.) https://pubs.acs.org/doi/abs/10.1021/acs.jpca.8b02432